FDA panel recommends making opioid overdose antidote available over the counter
A drug that can rapidly reverse opioid overdoses could soon be available to anyone without a prescription.
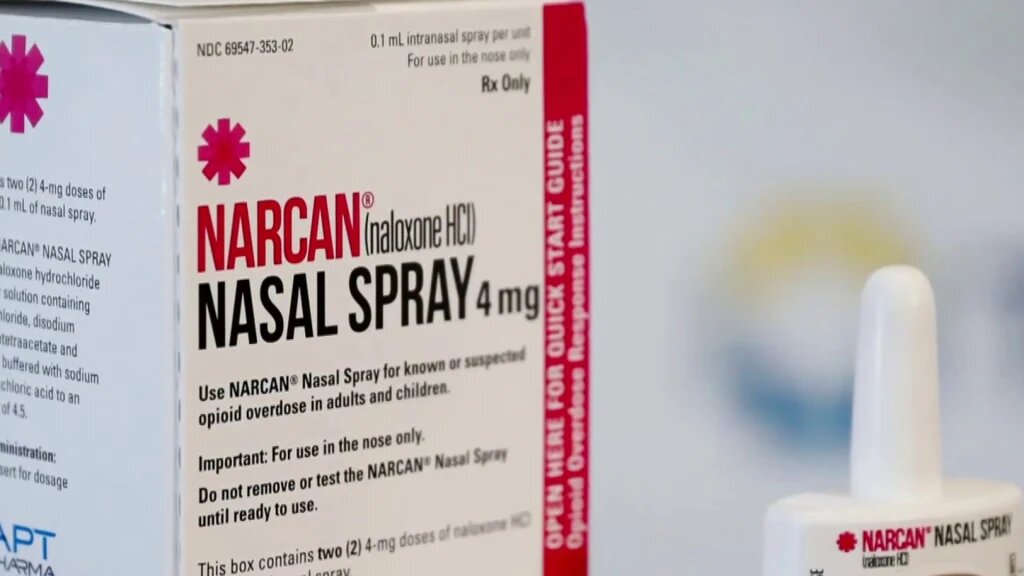
A Food and Drug Administration advisory committee on Wednesday voted unanimously to recommend that the agency allow a nasal spray version of naloxone, from Emergent Biosolutions, to be sold over the counter.
Naloxone, also sold under the brand name Narcan, is a medication that has been used for decades to quickly reverse the effects of an overdose from opioids, including prescription painkillers, as well as heroin and fentanyl. In November, the FDA asked drug companies that make naloxone to apply for over-the-counter approval.
Committee members said the move would save lives.
“For the sake of the public and saving lives, I believe this medication should be available over the counter as soon as possible,” Dr. Katalin Roth, a professor of medicine at the George Washington University School of Medicine and Health Sciences, said following the vote.
Drug overdoses are currently the leading cause of accidental deaths in the United States, according to the Centers for Disease Control and Prevention, killing more than 107,000 people in 2021. More than 80,000 of those deaths involved opioids.
"The risk of opioid overdose is far too great to prevent the drug from the OTC market," said Jennifer Higgins, the director of grants at the Center for Human Development, a nonprofit that provides community health services in Springfield, Massachusetts.
Currently, naloxone is offered only as a prescription medication, however, many states have created workarounds that allow people to get the drug directly from pharmacists. It can also often be found at community centers, local health departments and needle exchange programs.
Still, not all pharmacists keep the medication in stock and some patients may be shy about interacting with a pharmacist, Dr. Jody Green, an FDA official, said during Wednesday's meeting. And community centers that offer patients naloxone for free often have limited supply, she added.
The panel’s recommendation will now go to the FDA, which is expected to make a final decision by March 29. If the agency follows through on the approval, the drug could be sold in places like convenience stores, grocery stores — even vending machines.
As overdoses have become more common, so have prescriptions for naloxone: The number of naloxone prescriptions increased from about 359,000 in 2017 to 1.5 million in 2021, according to briefing documents published by the FDA. Read More…