Study Unveils Complexity of Zoonotic Transmission Chains
Researchers from the Complexity Science Hub (CSH) and the University of Veterinary Medicine Vienna have provided new insights into the intricate interactions involved in zoonoses, diseases that are transmitted between animals and humans. Affecting over two billion people worldwide each year, these diseases pose a significant public health concern. The study, titled "A One Health framework for exploring zoonotic interactions: a case study," was recently published in Nature Communications.
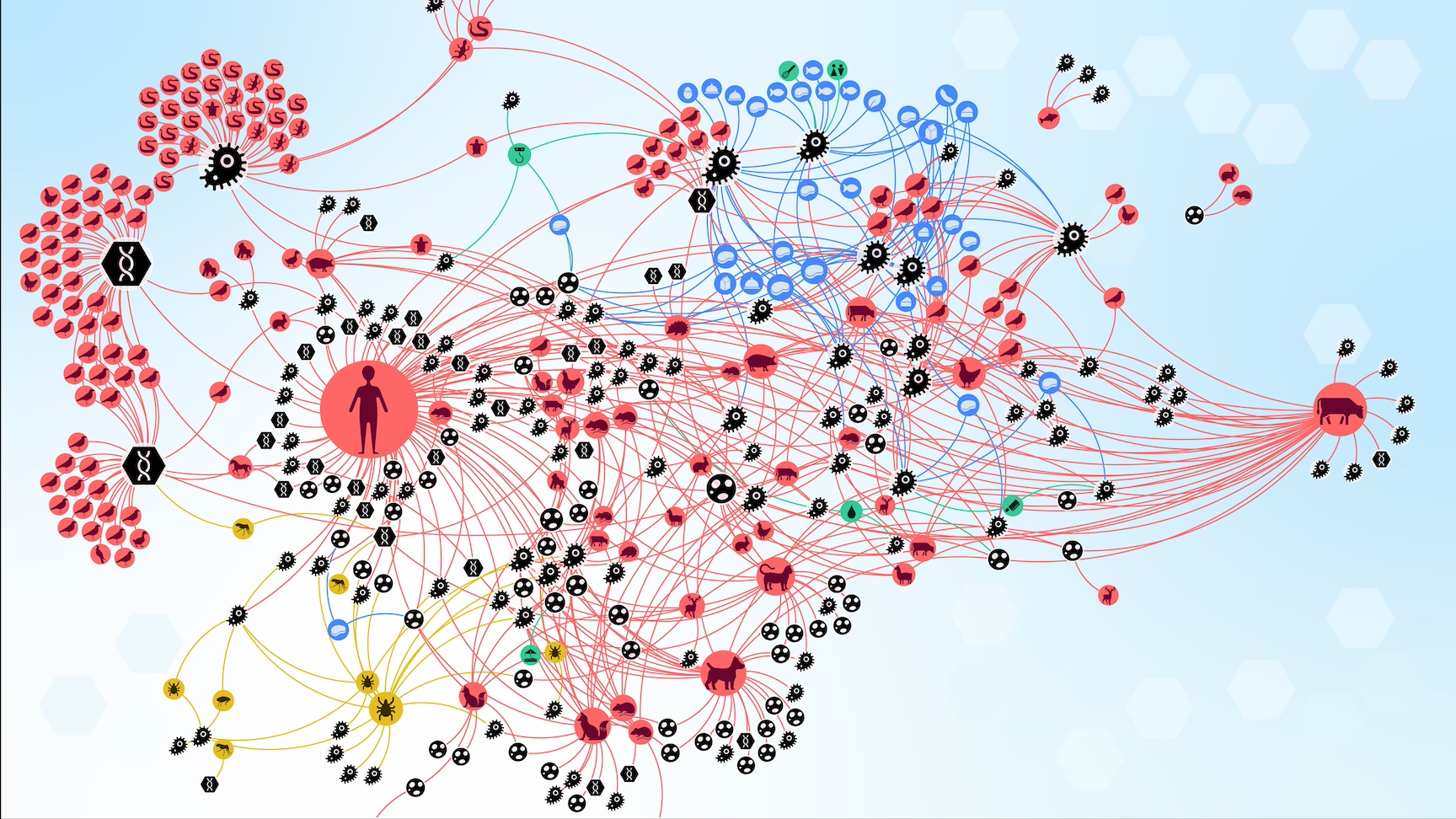
The research introduces the concept of a "zoonotic web," a detailed network mapping the relationships between zoonotic agents, their hosts, vectors, food sources, and the environment. This framework aims to enhance the understanding and management of zoonotic risks through a holistic approach.
Epidemiologist and CSH researcher Amélie Desvars-Larrive explains the importance of this comprehensive approach: "Zoonotic diseases, which can be transmitted between animals and humans, are a significant public health concern. Our study highlights the importance of a holistic approach to understanding and managing these risks."
Transmission Pathways:
- Direct Transmission: Occurs through direct contact with infected animals' saliva, blood, urine, or feces. Examples include:
- Rabies: Transmitted through bites.
- Cat Scratch Disease: Transmitted through scratches.
- Skin Fungi: Transmitted through skin contact.
- Indirect Transmission: Involves vectors like arthropods or contaminated environments and surfaces. Examples include:
- West Nile Virus: Transmitted by mosquito bites.
- Tick-borne Encephalitis: Transmitted by tick bites.
- Contaminated Food and Water: Potential routes of infection for various zoonoses.
Zoonotic Web
The researchers created a "zoonotic web" based on a systematic literature review of documented interactions between zoonotic sources and pathogens in Austria from 1975 to 2022.
This web includes the complex interactions between hosts, pathogens, and environmental factors.
The team identified six distinct communities of zoonotic agent sharing in Austria. These communities are influenced by highly connected infectious agents, proximity to humans, and human activities.
The community comprising humans, domesticated animals (e.g., dogs, cats, sheep, cattle, and pigs), and species adapted to human environments (e.g., house mice) shares the most zoonotic agents.
The study underscores the complexity of the animal-human-environment interface in zoonotic disease transmission. Understanding these intricate networks is crucial for developing effective prevention and control strategies.
As co-author Anja Joachim highlights, studying a single aspect of zoonotic transmission, such as the presence of a parasite in cat feces, doesn't provide the complete picture. The novel approach taken by Desvars-Larrive and her team goes beyond simple host-pathogen interactions to consider other sources of infection, such as contaminated environments and food.
Future Directions
The results of the analysis are presented in a dashboard created by CSH data visualization expert Liuhuaying Yang, providing an accessible tool for further research and policy development.
The researchers advocate for more comprehensive and integrative models that consider all potential sources and routes of zoonotic pathogen transmission.
This study represents a significant step forward in the understanding of zoonotic diseases. By developing the concept of the "zoonotic web," the researchers offer a powerful tool for dissecting the complex transmission chains that underlie these diseases. The holistic approach advocated by the study can potentially lead to more effective strategies for preventing and controlling zoonotic infections, ultimately protecting public health on a global scale.



