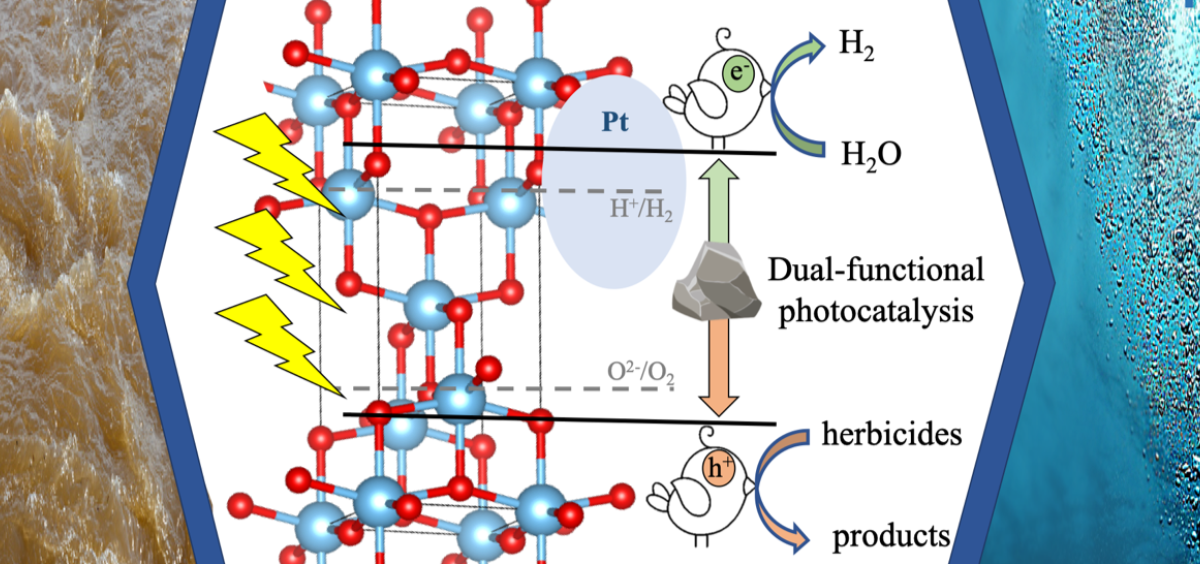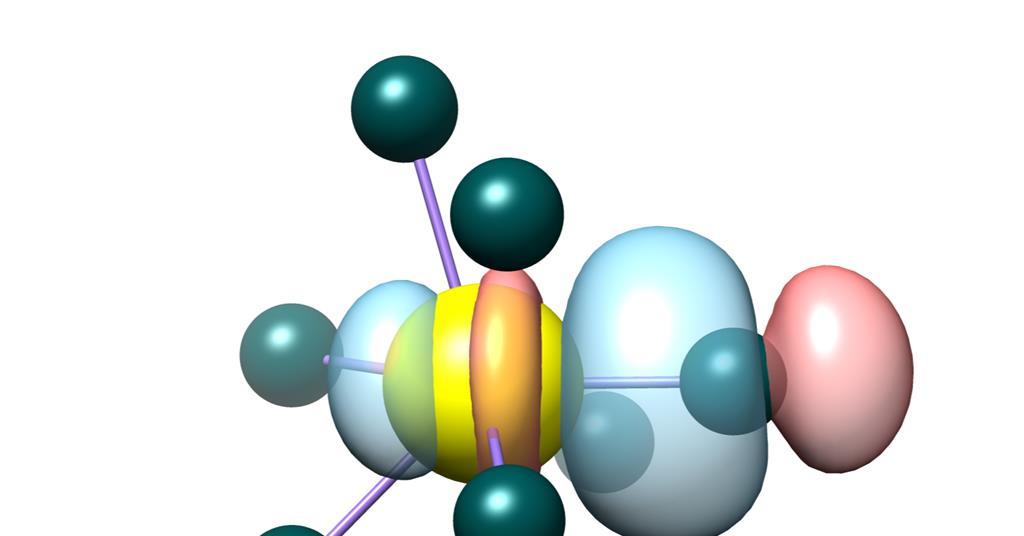
Redefining Secondary Bonding Interactions in Chemistry
Secondary bonding interactions, including hydrogen bonding, have long been a cornerstone of chemistry. However, the current definitions of these interactions have been criticized for being unclear and inconsistent. A new study proposes a revised framework for understanding secondary bonding interactions, aiming to end the confusion and provide a more accurate description of these crucial chemical forces.
The Limitations of Current Definitions
The current definitions of secondary bonding interactions, such as hydrogen bonding, halogen bonding, and π-π stacking, are often based on simplistic models that do not accurately capture the complexity of these interactions. These definitions can lead to confusion and inconsistencies in the classification of secondary bonding interactions.
A New Framework for Secondary Bonding Interactions
The proposed framework introduces a new set of definitions for secondary bonding interactions, based on a more nuanced understanding of the underlying chemical forces. The new definitions take into account the electronic and geometric characteristics of the interacting molecules, providing a more accurate and consistent description of secondary bonding interactions.
Implications of the New Framework
The proposed framework has significant implications for the field of chemistry, as it provides a more accurate and consistent description of secondary bonding interactions. The new definitions and classification system will enable researchers to better understand and predict the behavior of molecules, leading to advances in fields such as materials science, pharmaceuticals, and biotechnology.
The new framework for secondary bonding interactions represents a major step forward in the field of chemistry. By providing a more accurate and consistent description of these crucial chemical forces, the framework will enable researchers to better understand and predict the behavior of molecules, leading to advances in a wide range of fields.